JoAnne Flynn studies cells being grown in the laboratory.
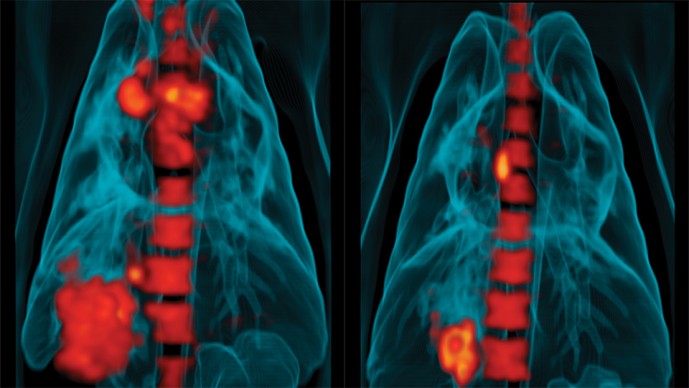
3D reconstructions of PET/CT scans generated by Young's collaborator JoAnne Flynn showing the lungs of a tuberculosis-infected monkey. Metabolically active areas are colored in red and are reduced after treatment with an anti-tuberculosis drug. These scans are being used by Young. Flynne and Barry to understand latent infection and find better ways of treating tuberculosis. Scans courtesy of Dr. JoAnne Flynn
Douglas Young, Imperial College London, United Kingdom
Two years into the Grand Challenges in Global Health grant to find new drugs for tuberculosis, Douglas Young, the project's principal investigator, received a Christmas card with a set of surprising photos. What he saw has helped set in motion a new understanding underlying the search for treatments of tuberculosis (TB), the respiratory illness that kills about 1.4 million people a year.
"I realized then that we were dealing with a more dynamic disease than we had thought," recalls Young, then a veteran TB researcher at Imperial College in London.
At the time, in late December 2006, Young was heading up a global team of about 100 researchers jointly funded with $10 million from the Bill & Melinda Gates Foundation's Grand Challenges in Global Health program, complemented by $10 million from the Wellcome Trust. The project's goal was to develop drugs to prevent full-blown disease in individuals who are infected by Mycobacterium tuberculosis but are asymptomatic - a condition called latent TB infection, or LTBI. An estimated two billion people have LTBI. About 10 percent of them will develop active TB, which can have a 50 percent mortality rate if untreated.
A symptomless carrier of the bacterium can be detected with a skin or blood test, and the infection can be cleared with nine months of drug therapy. However, it is impractical to treat everyone in this category, and the lengthy regimen makes adherence a challenge. Therefore, the goals of the Grand Challenges in Global Health project were to identify those with LTBI most likely to become sick, and develop drugs that can more rapidly wipe out the bacterium before it can progress to a potentially lethal stage.
Drugs that can eradicate the bacterium in a latent infection in a shorter time than current treatments would have a "major impact on TB worldwide," Young says. Because drug therapy can take nine months, about 20 to 30 percent of patients stop therapy before they are cured. In a scientific review article published in 2009, Young and two co-authors wrote that in order to meet the United Nation's goal of a 50 percent reduction in TB by 2050 "there will need to be interventions that reduce the percent of infected individuals who progress to clinical disease."[1]
"So our idea was to do fundamental research needed to understand how the bacteria survive in the latent mode," says Young. "I thought here's a formidable problem that requires formidable resources," Young says. "It seemed worthy of a grand challenge."
The holiday card's contents made clear the challenge was different than initially anticipated. Inside were photos of X-ray-like images of the lungs of a TB patient in Busan, South Korea produced by a colleague working on the project, Clifton Barry III, head of the TB research unit at the U.S. National Institutes of Allergy and Infectious Diseases. Barry had scanned the patient's lungs with an imaging technique that combined computed tomography (CT) and positron emission tomography (PET) technologies. The dual scanners can reveal structures inside organs and also gauge biological activity occurring within tissue with unprecedented detail.

The images showed glowing islands of various shapes and sizes speckled throughout the patient's lungs. Young immediately realized that each of these likely represented an inflamed lesion where the patient's immune system cells had walled off invading TB microbes. Young had expected each scanned lesion to emit about the same amount of light, or metabolic activity, which would support the idea that bacteria in latent lesions all had a similar biological profile. Instead, the inflammations radiated a wide variation of cellular energy.
"The patient had an amazing array of these lesions throughout both lungs. At that point we didn't even know if anything would be visible by PET," Barry remembers. By detecting how much of a tracer of radioactive glucose injected into the patient was absorbed by cells, the PET camera picked up the degree of activity in the inflamed region. Some lesions under the PET glowed bright white or yellow, indicating "hot" spots where an intense fight likely was taking place between bacteria and immune cells. Other lesions were "cold," appearing pink or dark yellow, suggesting the bacterium was under control or had been killed by the body's defenses. "It was a real eye opener for us." says Young. "Until then, we thought the lesions all would register a similar amount of activity, that latency was one biological state and did not have so much variation. It changed our whole vision of the pathology of TB."
Several months later, after the patient received drug therapy, "some lesion types appeared to get colder and begin to resolve while others got hotter." says Barry. "This spectrum of type and response rates was born out as subsequent patients accumulated - at a rate of two or three a month - and we really started to appreciate how variable individual lesions were."

Positron emission tomography (PET) coupled to a computerized tomography (CT) scanner is used to visualize small tuberculosis lesions in the lung, revealing the heterogeneity of the disease.
These first, real-time images of TB lesion activity within a living individual's infected lung, and dozens more periodic PET/CT scans of patients and laboratory monkeys over the next few years, produced "a major paradigm shift in the way we think about TB," says JoAnne Flynn, a researcher at the University of Pittsburgh recruited to the Grand Challenges in Global Health project by Young and Barry.
Barry's images showed that activity in specific lesions rises and falls in patients over time and in response to drug therapy. Flynn showed in PET/CT images in non-human primates that lesions visualized in early stages of latent disease behaved in a similar fashion.
"Now the whole field accepts the notion that a TB infection isn't binary - active or latent - but exists as one disease with a wide spectrum of activity," Flynn says. It was as if each lesion was its own "little world."
As this newer understanding of TB emerged, "we began to realize that a separate drug to attack bacteria residing in one specific latent state wasn't going to work because there wasn't just this one type of latency," she says.
This view of the infection is helping inform the current TB Drug Accelerator, a program begun by the Bill & Melinda Gates Foundation in 2007 to promote greater collaboration among academic institutions and pharmaceutical companies. In 2012, the Gates Foundation provided $20 million to fund projects at seven research institutions with the understanding they and eight pharmaceutical companies - AstraZeneca, Merck, Eisai, Eli Lilly, Abb Vie, Bayer, GlaxoSmithKline and Sanofi will share their data and findings. Its goal, over a ten-year period, is to develop a three-drug combination therapy that can reduce treatment to one month.
To reach this objective, the collaboration is investigating the spectrum of lesion types in active disease first identified by the Grand Challenges in Global Health project, says Ken Duncan, who oversees the program at the Gates Foundation. "We hypothesize that agents that can kill the bacterium in all the various micro-environments that exist within a host would more rapidly eliminate the persistent populations and lead to a more rapid cure." says Duncan.
The understanding developed by Young's Grand Challenges team that nodules of TB are so diverse is now the central theme of drug research, says Barry. "The next quantum leap in TB drug development will come from identifying a combination of drugs that complement each other in their ability to penetrate the range of lesions found in the spectrum of TB disease, and their potential to kill all bacterial subpopulations present in these lesions."[2]
When Young and his team first undertook their search for new drugs in 2005, funded by the Grand Challenges in Global Health program, the prevailing notion was that all TB bacteria that resided in latent form had similar attributes.
Small, latent tuberculosis lesions can now be visualized in the lung by using a fluorescent tracer that labels metabolically active cells making them glow red, and overlaying images taken using PET and CT (shown).
These characteristics enabled the bacteria to exist in a low oxygen, or hypoxic, environment with few other nutrients and walled off from the rest of the lung within nodules produced by inflammatory white blood cells.
To the researchers this meant the bacteria in asymptomatic persons were biologically different from those that caused active disease. The thinking back then was that active disease erupts among those with LTBI when previously dormant bacteria emerge from latent lesions, perhaps when a person's immune system is stressed by another infection, such as HIV. Research also suggested that existing drugs took a long time to work because they couldn't breach the exterior of lesions where immune cells had become calcified over time. In addition, antibacterial drugs mostly work by blocking the formation of cell walls when a bacterium replicates. The TB microbe within hypoxic lesions was thought to be non-replicating, so new cell walls weren't being built and therefore weren't a target for those drugs.
Young's Grand Challenges team decided to attack the problem initially from several fronts. Most notably, it began analyzing lesions removed from patients, probing the material for the presence of activated genes the latent bacterium might be employing to sustain itself in the inhospitable environment. The idea was to employ new techniques pharmaceutical company researchers were using to identify molecules involved in a disease pathway and then block them by screening agents from chemical libraries. The team guessed such bacterial genes likely produced proteins such as enzymes that supported life within the hypoxic environment. Inactivate those enzymes, they figured, and the bacteria can't survive. But after about a year or so, this effort, "didn't get us anywhere," Young says.
At the same time, as a partner in the Grand Challenges in Global Health project, the Swiss pharmaceutical company, Novartis, began an industrial-sized, high-throughput screening of compounds in its chemical library for activity against proteins the TB bacterium produced to help sustain itself in a latent condition. Novartis found agents that inhibited the molecules in laboratory tests. However, none were effective in killing latent bacteria, suggesting the microbe had other ways to stay alive in a quiet mode, Young says.
It was about this time, two years into the Grand Challenges in Global Health grant, that Barry produced his first PET/CT images. He then turned to Flynn, who had been developing an animal model to study TB in cynomolgus macaques. Flynn and Barry sought and received an additional grant from the Gates Foundation to build a PET/CT machine to scan the lesions in the macaques. "That changed our world," says Flynn, because now the researchers were able to watch changes in individual lesions in infected non-human primates over time and in response to a variety of drug regimens.[3],[4]
"We would never have had the support or the enthusiasm to do this in animals if we hadn't seen the human scans first." says Barry.
I thought here's a formidable problem that requires formidable resources,"Young says. It seemed worthy of a grand challenge."
In one set of experiments using PET/CT, Flynn produced images showing that existing TB drugs do reduce lesion activity. When the animals were exposed to a low dose of bacteria, half developed active disease, half had LTBI. When those with LTBI were administered a drug that blocks the activity of immune cells, those with latent TB developed active disease,[5] clear evidence the immune system was keeping certain bacterial populations at bay.
This technique allowed Flynn to trigger reactivation of the infection and test a drug's effectiveness more quickly than waiting for latent pockets of the disease to reactivate over time on their own. In one study, LTBI animals received six months of the drug isoniazid, which humans get for nine months to treat the disease. When immune cells were suppressed after the six-month regimen, none of the animals' latent infections, as gauged by the PET/CT scans and measurements of bacterial burden, reactivated.[6] "These experiments show us we can monitor how well new drugs work against lesions in people with latent and active disease," says Flynn. While Flynn received research support from the Gates Foundation, she also has grants from the U.S. National Institutes of Health and other funders.
In the same set of experiments, Flynn showed that a drug called metronidazole, when combined with isoniazid and another antibacterial, rifampicin, shut down lesion activity in just two months. Metronidazole was added because it works against bacteria in low-oxygen environments. A related, potentially more effective and safer compound, PA-824, is now in early clinical trials.[7] More recent studies have found that not all latent "micro-environments" have low levels of oxygen, further reinforcing the new understanding of the variable nature of latent infections.
Robert Wilkinson, a researcher at the University of Cape Town, is conducting similar PET/CT studies in South Africa among people in close contact with TB patients and very likely carrying a latent infection. Wilkinson's experiments are designed to replicate Flynn's research in humans to show that the scanner can gauge the impact of drugs in test subjects.
Wilkinson says PET/CT scans may make it possible to evaluate the impact of new drug therapies on lesion activity much quicker, and at a lower cost, than clinical trials that would have to follow many thousands of patients for years to see if new drugs prevented LTBI from becoming active. PET/CT could also play a role in drug trials for active disease if it provides an accurate measure of the effect of new drugs in small clinical studies, Ken Duncan says.
New drug trials also can be significantly shortened if researchers could identify who among those with LTBI are at highest risk of developing active disease - one of the original goals of the Grand Challenges in Global Health grant. Only those individuals would get enrolled in drug trials.
"So, what did we learn?'' asks Young. "Both latent and active disease involves a spectrum of lesion activity. Drugs that will shorten treatment need to work against bacteria existing within that entire spectrum. With PET/CT, we developed a way to measure the effectiveness of new drugs against bacteria that exist throughout the spectrum."
THE SCIENCE:
Discovering the intra-host heterogeneity of tuberculosis infection
A proportion of individuals with latent tuberculosis infection (LTBI) is at risk of developing active disease and thus requires preventative therapy. Identifying this subclinical population is currently not possible, so Young and colleagues have been studying the underlying biology of LTBI. They, and others, have discovered that tuberculosis is a highly heterogeneous disease, which has shifted the view from disparate latent and active infection states to a dynamic spectrum between the two that has important implications for treatment.[8]
Individuals infected with Mycobacterium tuberculosis present granulomatous lesions containing the bacteria and host immune cells. JoAnne Flynn and Clifton Barry, colleagues of Young working on the project, and others have shown that these lesions are highly heterogeneous in terms of their composition, metabolism, and microenvironment. They discovered that the formation and progression of individual lesions within the same host (humans and macaques), and in response to therapy, is also widely variable.[3], [9], [10], [11] This variability is thought to be due at least in part to the local immune environment of individual lesions, and overall disease outcome is the net result of all the local molecular and cellular interactions between host and pathogen.[12] Heterogeneity of host-pathogen interactions within the same host is being increasingly accepted as a common characteristic of other infectious diseases. Understanding how local factors influence drug response will aid in the search for more effective treatments.[13]
REFERENCES:
[1] Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 17, 183 (2009).
[2] Dartois V, Barry CE 3rd. A medicinal chemists' guide to the unique difficulties of lead optimization for tuberculosis. Bioorg Med Chem Lett. 23, 4741 (2013).
[3] [a],[b] Lin PL, Coleman T, Carney JP, Lopresti BJ, Tomko J, Fillmore D, Dartois V, Scanga C, Frye LJ, Janssen C, Klein E, Barry CE 3rd, Flynn JL. Radiologic responses in cynomolgous macaques for assessing tuberculosis chemotherapy regimens. Antimicrob Agents Chemother. 57, 4237 (2013).
[4] Russell DG, Barry CE 3rd, Flynn JL. Tuberculosis: what we don't know can, and does, hurt us. Science 328, 852 (2010).
[5] Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, Grieser H, Chiosea I, Voitenek NN, Capuano SV, Klein E, Flynn JL. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 62, 340 (2010).
[6] Lin PL, Dartois V, Johnston PJ, Janssen C, Via L, Goodwin MB, Klein E, Barry CE 3rd, Flynn JL. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc Natl Acad Sci U S A 109, 14188 (2012).
[7] https://clinicaltrials.gov/ct2/show/NCT02256696
[8] Barry CE 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 7, 845 (2009).
[9] Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, Via LE, Barry CE 3rd, Klein E, Kirschner DE, Morris SM Jr, Lin PL, Flynn JL. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 191, 773 (2013).
[10] Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, Sacchettini J, Fortune SM, Flynn JL. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med. 20, 75 (2014).
[11] Coleman MT, Chen RY, Lee M, Lin PL, Dodd LE, Maiello P, Via LE, Kim Y, Marriner G, Dartois V, Scanga C, Janssen C, Wang J, Klein E, Cho SN, Barry CE 3rd, Flynn JL. PET/CT imaging reveals a therapeutic response to oxazolidinones in macaques and humans with tuberculosis. Sci Transl Med. 6, 265ra167 (2014).
[12] Bumann D. Heterogeneous host-pathogen encounters: act locally, think globally. Cell Host Microbe 17, 13 (2015).
[13] Lenaerts A, Barry CE 3rd, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev. 264, 288 (2015).



