David Baltimore in the lab.
ENGINEERING IMMUNITY AGAINST HIV AND OTHER DANGEROUS PATHOGENS
- Jan 1, 2016
- Download Retrospective

Alejandro Balazs in the lab. Alex was a postdoctoral researcher in the Baltimore lab and a key member working on the HIV vaccine project.
David Baltimore, California Institute of Technology, United States
After thirty years of failure in the hunt for an HIV/ AIDS vaccine, researchers are closing in on a promising new approach.
Two similar vaccines with encouraging early results in animals are emerging from university laboratories. The two labs - one run by Philip Johnson at the University of Pennsylvania, and the other by Nobel Laureate David Baltimore at Caltech - conducted their research largely unaware of the other's efforts, and their vaccine candidates both result from the same basic scientific advances made in the past 10 years.
There is a cautious excitement growing among HIV/AIDS scientists about the vaccines that are scheduled for their first human testing in 2015. That's because the vaccines are the first to generate neutralizing antibodies, proteins produced by immune system cells, that can target HIV and sweep it out of the body before an infection can take hold. In one experiment mice engineered to have human-like immune systems were first given the vaccine and then exposed to HIV. The vaccine's neutralizing antibodies appeared to fully protect the lab animals from infection.[1] Not one of the dozen or so vaccines tested in well over 200 clinical trials since the late 1980s[2] has produced a similar effect in animals or humans. Preliminary clinical trials for potency and safety in a small number of volunteers started in 2014.
"It's a very innovative approach, but our enthusiasm needs to be tempered because a lot can happen in people that you don't see in animals," says Gary Nabel, chief scientific officer at Sanofi, the Paris-based pharmaceutical company. Nabel previously was head of the Vaccine Research Center (YRC) at the National Institutes of Health, which was set up by the U.S. government to shepherd new ideas for an AIDS vaccine. Under Nabel, the YRC began collaborating with the Baltimore lab to develop the vaccine for human testing.
"I'm enthusiastic about this idea," Nabel says. "But even if all goes well in these first trials, it will likely take years more research before these vaccines could be available."
One of the vaccines has surfaced after more than 12 years of research in Johnson's lab at the University of Pennsylvania, much of it conducted with little fanfare. The other vaccine emerged as a result of a series of scientific twists and turns at Baltimore's Caltech lab backed by US$13.9 million from the Gates Foundation's Grand Challenges in Global Health program.
In the kind of coincidence that often marks the emergence of a scientific advance, the Baltimore lab pursued its vaccine effort for two years without knowing that the Penn scientists had been working on the same idea. In fact, the Caltech lab received the Grand Challenges in Global Health grant to pursue a different and especially ambitious strategy devised by Baltimore to genetically engineer the human immune system to target HIV in ways it doesn't do naturally. The lab changed course dramatically in late 2007 when Alejandro (Alex) Balazs, a newly recruited post-doctoral researcher, convinced Baltimore, one of the world's most influential scientists, to abandon his initial strategy.
"I knew what I wanted to do originally, for which we received the Grand Challenges (in Global Health) grant, was going to be complicated. But I was committed to that approach," says Baltimore in a conference room outside his labs at Caltech where he is President Emeritus and a professor of biology. "Then Alex came along and forced me to think there might be a better way to get at the same goal. Alex deserves credit for his idea, for making it work, and for persuading me to change direction."
The new vaccines aren't really vaccines, not in the traditional sense at least. Classical vaccines, from the very first against smallpox in the late 18th century, are composed of either killed or weakened versions of a virus or, more recently, genetically engineered proteins that sit on the virus's outer surface envelope. The polio vaccine is a killed or neutered form of the poliovirus. The hepatitis B vaccine is a lab-created version of an envelope protein called an antigen. Each of these approaches work by awakening the immune system as if the virus itself had invaded the body. The vaccines produce neutralizing antibodies that lay in wait to ambush the real virus soon after an infection, as well as stirring the production of T cells that are also part of the body's disease defenses.

But none of the experimental AIDS vaccines produced to date - all composed of laboratory-made antigens to avoid using even a deactivated or killed version of the virus that might cause disease - have spurred an immune response potent enough to counter an HIV infection. The antibodies triggered by these test vaccines have been ineffective against the mutant forms of the virus that arise soon after an infection.
The Johnson and Baltimore vaccines circumvent this problem: they aren't designed to stimulate a protective immune response in the conventional way. Instead, the Penn and Caltech labs have developed similar ways to introduce potent HIV neutralizing antibodies detected in the blood of HIV-infected people. The antibodies have been discovered over the past decade by researchers painstakingly sifting through the blood of thousands of HIV-infected individuals.
The Penn and Caltech vaccines employ new technologies to deliver the newly identified antibodies into the body. The research labs accomplished this, at least in animal studies, by inserting genes for the antibodies into a virus, called a vector, that doesn't cause human disease. When the virus, known as "adeno-associated virus," or AAV, is loaded with the gene and injected into the animals' muscle, cells there are directed to secrete large amounts of the antibody into the bloodstream. In humanized mouse tests, these antibodies were found to circulate in large numbers for the animal's entire life.
"If that protection can be replicated in people, we'd have a one shot vaccine," Baltimore says.
The Baltimore lab's vaccine candidate is scheduled for its first human testing in early 2016. A clinical trial of several versions of Penn's candidate, in collaboration with the International AIDS Vaccine Initiative (IAVI), enrolled four volunteers for each of several vaccine doses in the United Kingdom in 2014. "It's the first test to see if the antibodies are expressed in humans at levels that may be protective," says Wayne Koff, senior vice president and chief financial officer at IAVI. The results of that test, to determine the appropriate dose level, were scheduled for release in 2015.
For Baltimore, if creating a potential "one-shot" vaccine wasn't a total surprise, it certainly wasn't what he set out to achieve as part of the Grand Challenges in Global Health grant. Baltimore, who still runs a large and active lab at age 77 after years running Rockefeller University and Caltech, began his search for an AIDS vaccine soon after the virus was identified. "I had been thinking since 1986 about ways of making an HIV vaccine and had not had a terribly useful idea," he says. "I watched the field flailing around and failing. It was a question that was uppermost in my mind."
As early as 2002, Baltimore began thinking of a solution, though it entailed some pretty complicated molecular biology, something he had been adept at his entire career. Back in 1975, at age 37, Baltimore shared the Nobel Prize for Physiology for the discovery of reverse transcriptase, an enzyme critical for the replication of so-called retroviruses like HIV. One class of anti-HIV/AIDS drug, for instance, works by blocking the virus's reverse transcriptase.
The vaccines are the first to generate neutralizing antibodies that can target HIV and sweep it out of the body before an infection can take hold.
In 2002, Baltimore's lab did a series of experiments that re-programmed immune cells called T cells to have characteristics they didn't normally have. They did this by creating new protein receptors on the T-cells' surface. The researchers delivered the genes for the receptors into test animals by inserting them inside a vector called a lentivirus. "It worked, it worked really well," Baltimore says. Baltimore says the Grand Challenges in Global Health grant gave him enough money to try to repeat the T cell reprogramming in B cells, the blood cells that produce antibodies. The effort was costly because it involved experiments using large numbers of expensive laboratory mice bred without an immune system. These mice could be given cells that created within the rodents a "humanized" immune system, providing a human-like research model to test his idea.
By 2007 the lab ran into several problems. Mostly, it was unable to get enough DNA into the lentivirus to trick the B cells into making large amounts of the antibodies. At that point, Baltimore recruited Alex Balazs to the lab. Balazs had done his graduate work in the lab of Richard Mulligan at Harvard Medical School. For many years, Mulligan has been researching ways to insert genes into viral vectors to treat a variety of diseases, an approach referred to as gene therapy.
Molecular biology was a key strategy in this project, which involved engineering broadly neutralizing HIV antibody genes into specialized vectors.
At first, Balazs tried tinkering with the mechanics of the vector. Then he got a better idea. "I remembered Mulligan said the AAV vector was often the best way to deliver genes," says Balazs. The AAV vector was especially good at expressing genes in animals, which Balazs thought might solve one problem the Baltimore lab faced. As Balazs wasn't familiar with AAV technology, he and several other members of the lab visited James Wilson, a gene transfer scientist who had developed a number of different AAV vectors. Wilson, also at University of Pennsylvania but not associated with Phil Johnson's lab, provided a week's worth of tutoring.
"All along I had been thinking that David's system was too complicated to become a usable vaccine, especially in the developing world," says Balazs. "I asked David why not just deliver the genes of the antibody instead of trying to get B cells to make the antibodies. The Gates Foundation was looking for a product and I worried about the complexity and the cost of David's approach."
As a result, the Baltimore lab made a crucial pivot. Instead of using the vector to try to reprogram B cells, they simply used the vector to deliver into the body genes that make the antibodies.
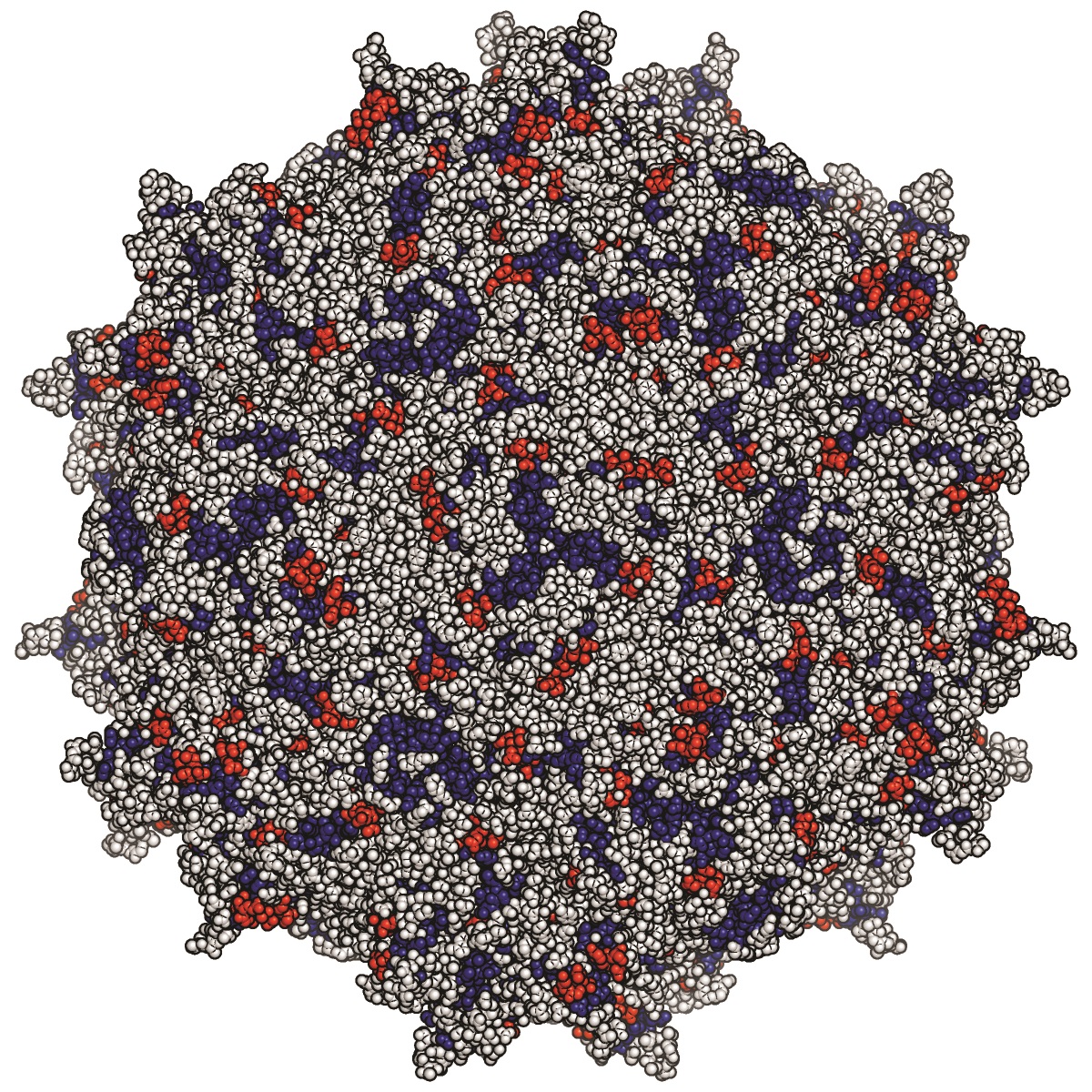
An illustration shows the crystal structure of the adeno-associated virus used to deliver broadly neutralizing antibodies as Vectored lmmunoProphylaxis against HIV.
Baltimore says at first, he was reluctant. "It forced me to think about just achieving a goal rather than the process to achieve it," says Baltimore. "With Alex's way we could reach the same goal with a simpler process." Baltimore also says that the Grand Challenges in Global Health had set timelines to achieve milestones, and already almost three years of the grant had passed without a usable vaccine in sight. Despite his desire to create a way to reprogram the immune system that could be used in fighting a variety of diseases, Baltimore says the grant's focus on a viable result made him rethink the best way to go. "The targets that were being set and the progress reports were new for me," says Baltimore. ''At first I didn't like the idea, but in the end it encouraged us to get out a product, not just a new technology."
For the next two years, Balazs focused on tinkering with the vector and testing different ways of producing larger and larger amounts of antibodies. They also began using increasingly more potent antibodies being developed at the NIH's Vaccine Research Center, and by scientists at Scripps Research Center led by Dennis Burton. At the same time, says Baltimore, the team realized they could simply inject the vector carrying the antibody genes directly into muscle, making a potential vaccine even simpler because it meant a shot could be administered to the upper arm.
In a series of tests in late 2009 and 2010, the lab found a vector-antibody pairing that worked. The AAV vector carrying a gene for a neutralizing antibody from the VRC protected the humanized mouse against HIV.[1] In addition, Baltimore says, experiments showed that "you could do a single injection into muscle of a mouse eight weeks old and a year and a half later it was still producing protective levels of the antibody." At about then the Grand Challenges in Global Health grant ran out and through the VRC new NIH funding was provided.
Meanwhile, Philip Johnson had begun working on a similar concept as far back as 2002.[3] He was using a version of the same vector – AAV - to deliver antibodies, and he also had discovered that the vaccine could be easily injected into muscle. In a set of experiments published that year, Johnson's lab inserted genes for a weak HIV antibody into an AAV vector and, by injecting mouse muscle tissue, showed that the antibodies continued to be present in the rodent blood for six months.
Over the following years, Johnson tried the concept on primates with immune systems more like humans, and who were susceptible to a virus similar to HIV. The breakthrough, Johnson says, was totally dependent on the emergence of neutralizing antibodies powerful enough to suppress an infection. In 2009, Johnson's lab reported that using AAV to deliver antibody-like genes against the primate virus resulted in total and long-lasting protection.[4] In the paper, Johnson's team wrote, "this strategy ... holds significant promise as a novel approach to an effective HIV vaccine." At that time, Johnson didn't actually insert genes for neutralizing antibodies into his vector because, unlike Balazs, his lab hadn't yet figured out how to get the entire gene for the antibodies into AAV. His lab accomplished that sometime soon afterwards.
The paper's publication was the first Baltimore and Balazs knew of Johnson's work. By then the Baltimore lab was already developing a vector to carry a neutralizing antibody. "Maybe we should have known what Johnson was doing," says Baltimore. "But we didn't."
Now the two teams are continuing on parallel tracks, using different antibodies and also different versions of AAV. In collaboration with IAVI, Johnson has since developed the AAV vaccine carrying genes for neutralizing antibodies discovered at Scripps. Johnson also is producing the AAV vector that the VRC will use in its planned clinical test of the Baltimore lab vaccine. The two vaccines produce different neutralizing antibodies, but both research teams say as newer even more potent antibodies come along they could be used instead.[5] "We're developing a second generation product that produces antibodies 10-fold more potent than last year," Johnson said in early 2014. "I expect the field to continue to progress."
Indeed, researchers say the gene delivery technique might be used in delivery of antibodies against other diseases for which there are no vaccines, such as malaria or tuberculosis. In January 2014, Balazs left Caltech and joined the Ragon Institute, a new research organization created to develop a vaccine against AIDS. There, Balazs has his own lab and hopes to continue working on the antibody-generating vaccines. One concern he and Johnson's lab are focusing on is potential safety issues related to the AAV vector. In addition, Balazs and other research teams are beginning to study whether the neutralizing antibodies could constitute a treatment for people already infected. "There's a great deal of work to be done." says Balazs.
THE SCIENCE:
Developing vectored immunoprophylaxis using broadly neutralizing antibodies to prevent HIV infection
David Baltimore and colleagues have been developing an alternative to conventional HIV vaccines, which have thus far failed to achieve broad protection in humans. The approach - vectored immunoprophylaxis - exploits rare broadly neutralizing HIV antibodies found in long-term-infected individuals. The corresponding antibody gene is engineered into a non-integrating adeno-associated virus (AAV) vector that is injected into the muscle where the antibody is synthesized and passively distributed to the circulatory system. Philip Johnson and colleagues showed feasibility of the method for HIV in immunodeficient Rag1 mice by engineering AAV to carry the broadly neutralizing antibody IgG1b12, only one of five identified at the time. Muscle-secreted IgG1b12 was detected in serum for the 6-month study duration, and could neutralize several HIV-1 strains in vitro.[3] They went on to show that antibody-like immunoadhesins with SIV specificity could also protect rhesus macaques from intravenous SIV challenge.[4]
Improved vectors were needed for expression of full-length antibodies at levels likely required for successful HIV protection in humans. Baltimore and colleagues incorporated the capsid of AAV serotype 8 into an AAV vector engineered with a newly created muscle-optimized promoter to drive expression of two different antibodies: b12, and the newly identified VRC01. The optimized vector produced higher serum antibody concentrations that protected mice from CD4 T cell depletion by high intravenous doses of laboratory HIV strains.[1]
As human HIV transmission typically occurs across mucosal membranes, they showed that intramuscular injection of the same AAV vector into bone marrow-liver-thymus humanized mice could also prevent mucosal transmission of REJO.c, a CCR5-tropic transmitted molecular founder HIV strain, which is similar to the strains that infect humans, even after repetitive intravaginal challenge.[6] This approach may be applied to other diseases for which vaccines have failed such as malaria.
REFERENCES:
[1] [a],[b],[c] Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481, 81 (2011)
[2] Esparza J. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine 31, 3502 (2013).
[3] [a],[b] Lewis AD, Chen R, Montefiori DC, Johnson PR, Clark KR. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol. 76, 8769 (2002).
[4] [a],[b] Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 15, 901 (2009).
[5] Schnepp BC, Johnson PR. Adeno-associated virus delivery of broadly neutralizing antibodies. Curr Opin HIV AIDS 9, 250 (2014).
[6] Balazs AB, Ouyang Y, Hong CM, Chen J, Nguyen SM, Rao DS, An DS, Baltimore D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 20, 296 (2014).



